CORROSION CONTROL
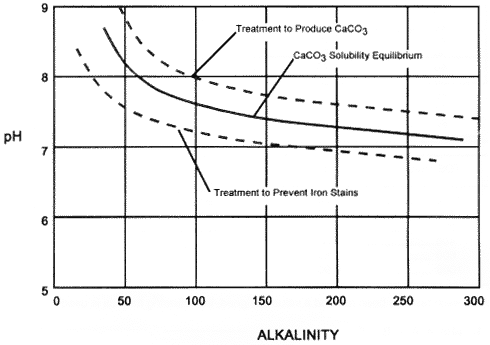
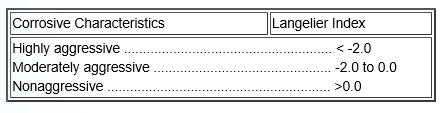
One of the best tests for determining the corrosivity of water is the Langelier Saturation Index (Ll). The Ll requires the following tests: Calcium hardness, total alkalinity, pH, total dissolved solids (TDS), and water temperature in centigrade. Once the tests have been performed they are assigned a value from the following tables. Notice that hardness and alkalinity (C and D) both use the same table.
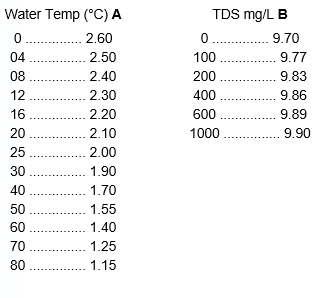
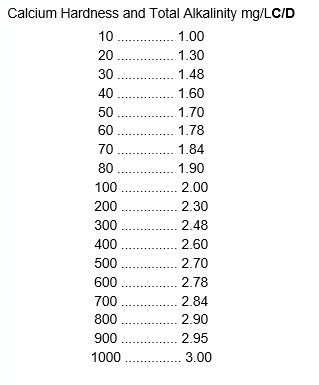
After each test has been run and a value assigned, the following formula is used.
- pHs = A + B – C – D
then - LI = pH actual – pHs